Dissolution and secondary mineral precipitation in basalts due to reactions with carbonic acid
Information
Journal of Geophysical Research: Solid Earth | 2017
Authors:
Shreya Kanakiya, Ludmila Adam, Lionel Esteban, Michael C. Rowe, Phil Shane
DOI: https://doi.org/10.1002/2017JB014019
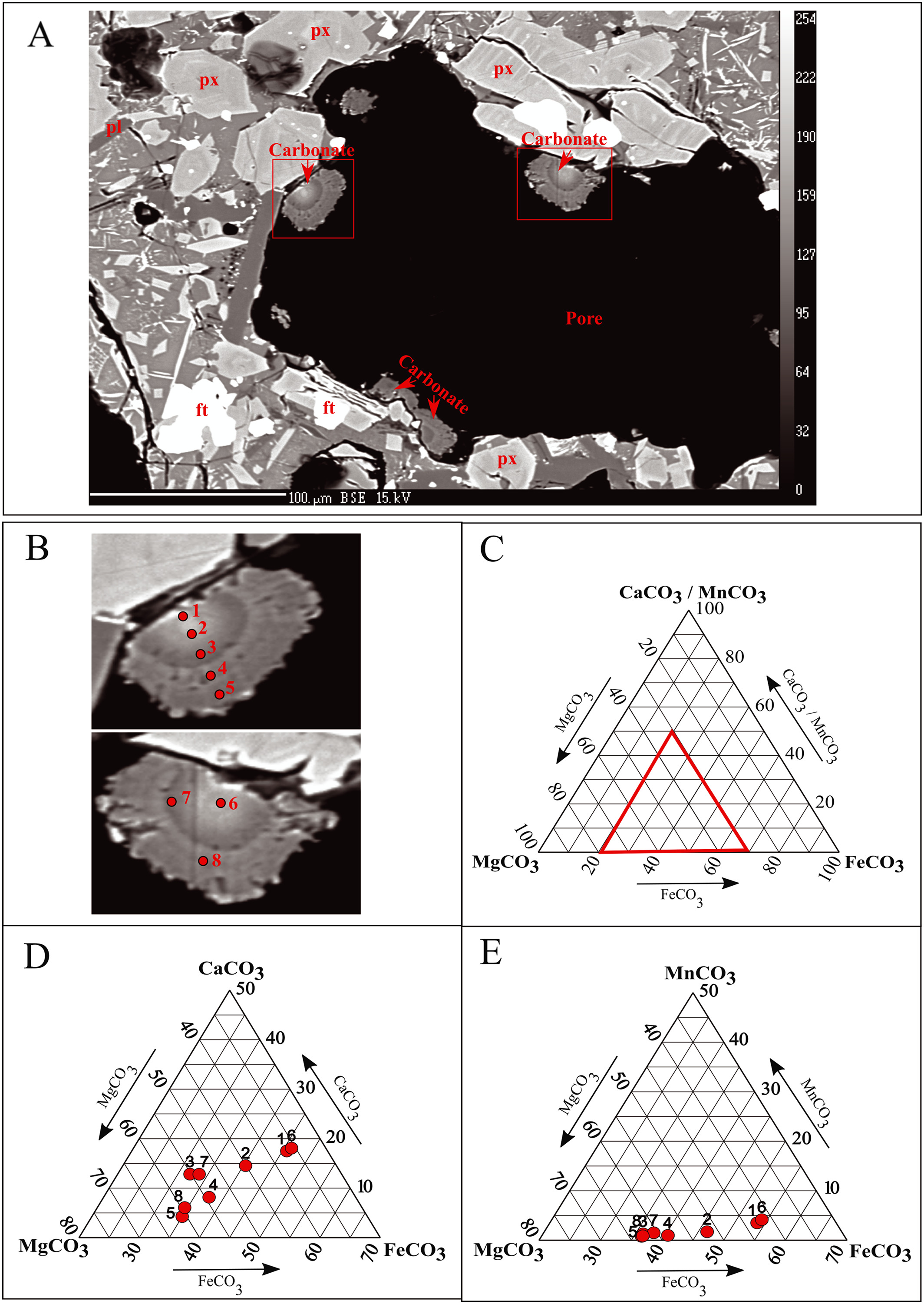
One of the leading hydrothermal alteration processes in volcanic environments is when rock-forming minerals with high concentrations of iron, magnesium, and calcium react with CO2 and water to form carbonate minerals. This is used to the advantage of geologic sequestration of anthropogenic CO2. Here we experimentally investigate how mineral carbonation processes alter the rock microstructure due to CO2-water-rock interactions. In order to characterize these changes, CO2-water-rock alteration in Auckland Volcanic Field young basalts (less than 0.3 Ma) is studied before and after a 140 day reaction period. We investigate how whole core basalts with similar geochemistry but different porosity, permeability, pore geometry, and volcanic glass content alter due to CO2-water-rock reactions. Ankerite and aluminosilicate minerals precipitate as secondary phases in the pore space. However, rock dissolution mechanisms are found to dominate this secondary mineral precipitation resulting in an increase in porosity and decrease in rigidity of all samples. The basalt with the highest initial porosity and volcanic glass volume shows the most secondary mineral precipitation. At the same time, this sample exhibits the greatest increase in porosity and permeability, and a decrease in rock rigidity post reaction. For the measured samples, we observe a correlation between volcanic glass volume and rock porosity increase due to rock-fluid reactions. We believe this study can help understand the dynamic rock-fluid interactions when monitoring field scale CO2 sequestration projects in basalts.
Highlights
-
Basalt-carbonic acid reactions precipitate ankerite minerals.
-
Porosity increases and rock elasticity decreases dueto these reactions
-
Rock dissolution, linked to volcanic glass content, dominates over carbonate precipitation.
Frequently Asked Questions
- Where can I find the samples used in this study?
-
The samples are at the Physics of Rock Lab at the University of Auckland.
- Where can I find the data from this study?
-
The authors have provided all data in this manuscript's tables and figures. You can access tabular data underlying specific figures by contacting me.